What's New
Compatibility update.
App Description
Stoichiometry Pro targets common stoichiometric tasks: balancing chemical reactions of unlimited number of components, finding molar masses, moles and masses of reactants and products and defining the limiting reagent, based on chemical formulas, stoichiometric coefficients, and any quantitative data provided.
The app analyses chemical reaction typed in “Reaction” field, balances it (if possible), finds molar masses and builds table with list of compounds (reagents and products coloured differently), for quantitative data input. All that triggered by pressing the = button.
Example 1: How many moles of H3PO4 are needed in order to obtain 2.0 g of NH4NO3, in the reaction:
H3PO4 + (NH4)2MoO4 + HNO3 = (NH4)3PO4(MoO3)12 + NH4NO3 + H2O
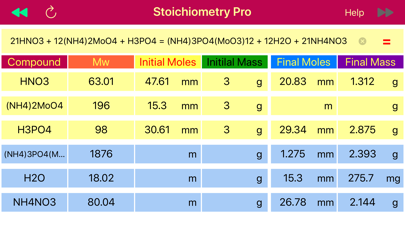
Solution: The first step is balancing chemical reaction and finding molar masses, since moles-mass conversion is needed. Reaction is balanced and all molar masses are calculated automatically by pressing the “=” button.
H3PO4 + 12(NH4)2MoO4 + 21HNO3 = (NH4)3PO4(MoO3)12 +21NH4NO3 + 12H2O,
The next step is to find the moles of NH4NO3 from the mass (2.0 g), and then convert them to moles of H3PO4 based on 1:21 ratio obtained from the balanced reaction. Stoichiometry application provides data table that comprised of masses and mole rows for data available before reaction initiated and obtained or left after the reaction is accomplished. When calculate button that pointing towards products, ▶▶ is pressed, the final masses and moles are calculated from the initially provided amounts of reactant and products. On the opposite, when the calculate button pointing left,◀◀, is pressed, then initial reactant and product amounts that would be required to achieve the provided final state are calculated. The later is exactly matches our situation, as we need to find the initial amount of H3PO4 from the final, known NH4NO3 mass. So we set the NH4NO3 mass (2.0 g) to the final mass field and press calculate button ◀◀ to automatically fill the table with all masses and moles for all components. Apparently, 1.19 mmoles of H3PO4 are required to get 2.0 g of NH4NO3.
Important Points:
Either moles or masses can be filled in, but if both are provided and they don’t match, then the moles value will have a preference.
For balancing redox reactions please refer to Electrochemistry application.
App Changes
- January 02, 2021 Initial release
- August 05, 2021 New version 1.7
- October 04, 2023 New version 2.0